Fluoride
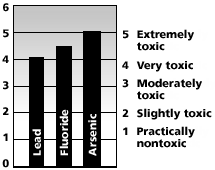
Fluoride (F+) is a constituent of many minerals. It is commonly added to public drinking water supplies in less enlightened parts of the world ostensibly for the prevention of tooth decay. Cities often maintain a level of 1.5 to 2.5 mg/L, but concentrations of about 5 mg/L are considered damaging to teeth. High levels of fluoride are often found in the waste water of manufacturing facilities where glass and steel are made. The organic fluorine called sodium fluoride is a by-product of aluminum manufacturing and is a main ingredient of rat poisons. Fluoride that results as a toxic waste by-product of aluminum and fertilizer manufacturing is the main source of the fluoride that is added to public water supplies. The EPA has established an MCL of 4 mg/L for fluoride. Fluoride is a powerful systemic poison, comparable to lead and arsenic.
Treatment: Fluoride can be reduced by anion exchange. It can also be reduced by filtration through activated alumina but is best removed by reverse osmosis (93 to 95%) and distillation. Removing Fluoride from Water Fluoride occurs naturally in some waters, but is frequently added to municipal water supplies because of a widely held belief that it prevents dental caries. Fluoride is more difficult to remove than most water contaminants. For practical purposes, there are three choices for removing fluoride from drinking water: distillation, reverse osmosis, and filtration through a special fluoride/arsenic reduction medium called activated alumina. Bone char carbon is used in some localities. Steam distillers remove a high percentage of fluoride, and virtually any good reverse osmosis units will provide fluoride reduction of 95% or better. Activated alumina, called AAL, removes fluoride well, but requires a relatively large bed and very slow flow rates. Some cartridge makers recommend flow rates in drinking water units of 1/4 gallon per minute or less for optimum performance for a drinking-water size cartridge. Because it requires such slow flow rates, AAL is not practical for whole house filtration. In fact, there is no suitable whole house treatment for fluoride removal at the present time. (Whole house reverse osmosis is not a practical solution for most situations.) Our feeling is that your best choice for whole house filtration is a high quality activated carbon filter. Although activated carbon is not normally recommended for fluoride reduction, it is known that under the right conditions carbon does remove some of the fluoride from tap water. And even if fluoride removal is minimal, carbon filtration makes significant other improvements by removing chlorine, chloramines, the by-products of chlorination, and chemicals in general. A special carbon called bone char is used in some parts of the world for fluoride reduction, but availability is limited. Standard carbons are made from such raw materials as coal, wood, and nut shells. Bone char carbon is made from animal bones. Go here for more specifics on fluoride removal.
|
|
The information below is specific to large fluoride removal applications, but it sheds light on residential application of activated alumina.
Activated Alumina Fluoride Removal Activated Alumina has long been considered the best technology for fluoride removal from aqueous solutions. It is currently used to treat potable water for municipal and household units as well as remediation of waste streams. The adsorptive process is simple requiring a flow rate across the media with a five minute empty bed contact time. Remediation and municipal fluoride removal systems normally require regeneration to make them cost effective. Regeneration is accomplished by a simple process whereby a dilute caustic solution is used to strip the adsorbed fluoride and other dissolved contaminants off of the surface of the media. The caustic step is followed by rinsing and then the AA is reconditioned with sulfuric acid. As some of the alumina can be dissolved during regeneration it is recommended to plan for periodic media “top-up”. REMEDIATION PROCESS: - Step 1: Regenerate with 1% NaOH, Caustic Solution The effectiveness of the media to adsorb fluoride is affected by a number of factors: Influent Fluoride Level: The capacity is bound by basic adsorption theory, the greater the concentration of fluoride, the higher the adsorptive capacity of the media. pH Level: The pH level also has a large impact on the ability to adsorb F. The optimum operating pH level is from 5.5 to 6.5. Adjustment (and readjustment, if necessary) is easily accomplished with the same solutions as regeneration. Contact Time: 5 minute Empty Bed Contact Time achieves the best balance of adsorption capacity and capital cost. Competing Ions: Fluoride is strongly attracted to activated alumina leaving few competing water contaminants. Silica, can reduce the fluoride adsorption capacity, although the silica effect is limited when pH adjustment is employed. Systems where silica levels are higher than 30 ppm may require more frequent and aggressive regeneration. Preconditioning Activated Alumina: The capacity of the activated alumina is greatly enhanced after conditioning. The first regeneration acts as a conditioning step. Note that the first treatment of non-preconditioned AA will yield a capacity 30% less than subsequent cycles after regeneration. Non Regenerative Systems: Activated alumina is also employed in some non regenerative applications. Source of the information above is IDS Water. Activated alumina used in residential drinking water units is not regenerated but replaced when its capacity is spent. Its lifespan depends on a number of variables, but most drinking water cartridges are good for at least a year if flow rates are kept low. |
|
|
|